Registered OHBM participant? Please rate this abstract here
Martin Tik1, Matic Princic1, Michael Woletz1, Anna-Lisa Schuler1, Christian Windischberger1
1Medical University of Vienna, Vienna, Austria
Introduction:
Transcranial magnetic stimulation is one of the most promising tools for treatment of depression. A consistent predictor for treatment efficacy is reflected in the anti-correlation between the subgenual ACC and the DLPFC before stimulation and an increase in connectivity afterwards as indicator for treatment response (Fox et al., 2012; Williams et al., 2018). We have shown in a large sample approach that offline TMS leads to a connectivity increase in ACC (Tik et al., 2017) to a specific resting-state component (RSN#17; Biswal et al., 2010).
To demonstrate the missing target-engagement during TMS we aimed to investigate (1) direct sgACC activation changes during stimulation and (2) TMS RS effects in RSN#17 on a single-subject level.
We employed an online 10Hz TMS/fMRI paradigm and resting-state fMRI immediately before and after, in order to predict treatment response in terms of connectivity changes on a single subject level.
Methods:
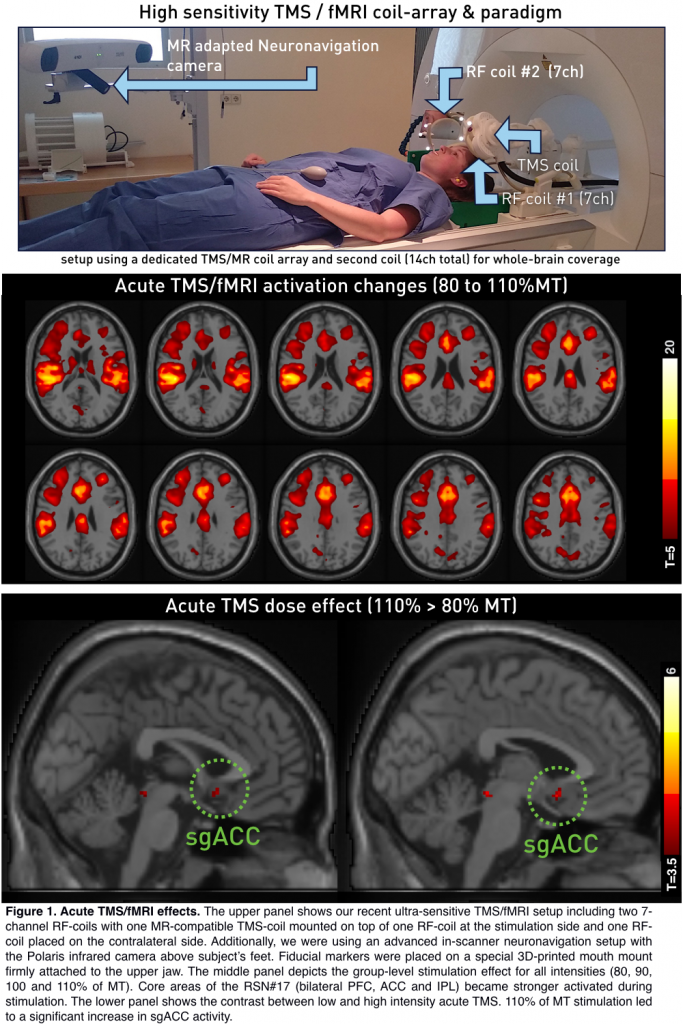
To investigate acute TMS/fMRI-effects, 14 right-handed subjects (6 male, age: 28 ± 3.9 years) were included in the experiment. The study was performed on a 3T Prisma scanner (Siemens, Erlangen, Germany) using a TMS/fMRI setup comprising the MagProX100 stimulator with an MRi-B91 MR-compatible TMS coil (Magventure, Farum, Denmark). Functional images during stimulation were acquired using the CMRR EPI sequence with a TR/TE=1000/38ms, 36 slices, 3 x 3 x 3mm3, 20% slice gap, MB-factor=4, delay in TR=305ms. The TMS/fMRI coil-array was positioned over the left DLPFC using an MR-compatible neuronavigation system. The stimulation target was at Brodmann area 46 (MNI-coordinate: -42, 28, 21). Concurrent TMS/fMRI was performed with a dedicated TMS/fMRI coil-array including one 7-channel surface RF coil with a TMS coil mounted on top. A second 7-channel RF-coil was placed on the contralateral side of the head in order to guarantee whole-brain coverage. TMS/fMRI consisted of triplets repeated five times for each of the four intensities adding up to a total of 60 pulses.
Six minutes of resting-state fMRI were acquired once directly before and once directly after concurrent TMS/fMRI (TR/TE=500/38ms, 24 slices, 3 x 3 x 3mm³, 25% slice gap, MB-factor=4).
Optical neuronavigation was performed throughout the whole measurement utilizing MR-compatible in-house built 3D-printed fiducial markers. This guaranteed optimal coil positioning surveillance throughout the whole setup. We performed seed-voxel analysis based on the RSN#17 by Biswal et al. (2010) for the resting-state measures (Fisher’s r-to-z transformed Pearson correlation coefficient) directly before and after concurrent TMS/fMRI.. Stimulation data analyses was performed using SPM12 with 4 task regressors representing stimulation intensities.
Results:
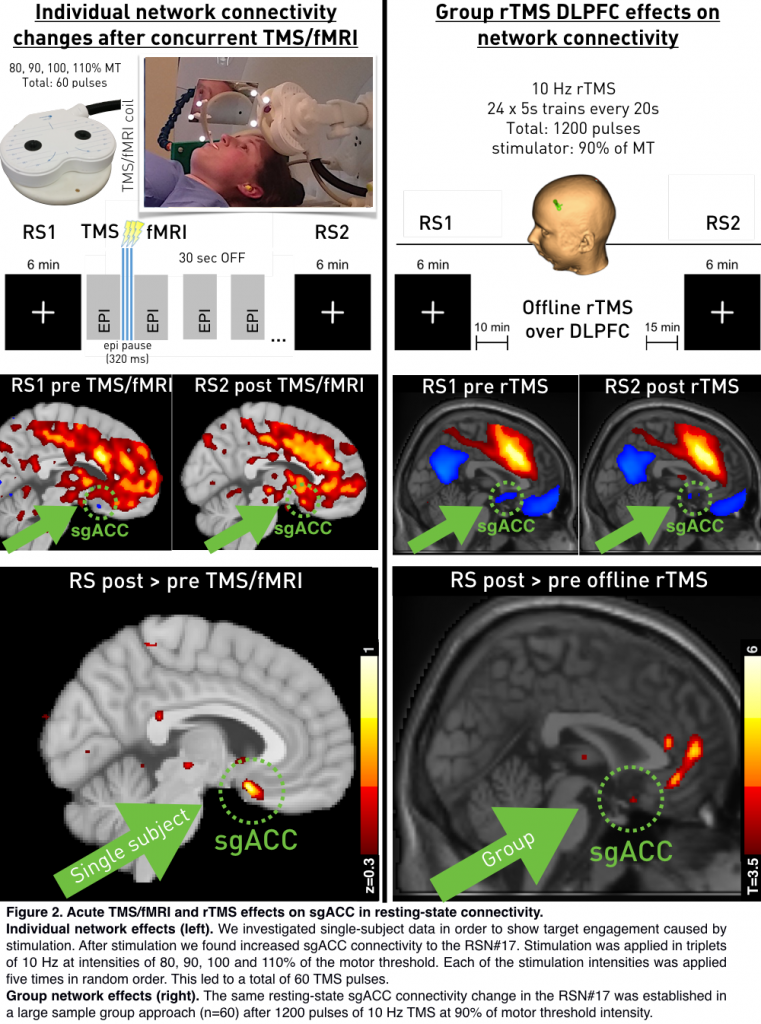
Single-subject connectivity between sgACC and the RSN#17 was anti-correlated (z=–0.3; Figure 2, bottom left). After concurrent TMS/fMRI connectivity of the subgenual anterior cingulate cortex to the IC#17 increased to become positively coupled (z=+0.37). The comparison between z-values pre and post stimulation revealed a specific connectivity change in the subgenual ACC.
The same effect was formerly shown after 1200 pulses of 10 Hz rTMS (Figure 2, bottom right). Regarding acute TMS induced BOLD-responses, concurrent TMS/fMRI led to activity increases in core brain areas of the RSN#17 (T-contrast over all intensities) and increased activity in the subgenual ACC for high intensity stimulation (110>80%).
Conclusions:
Here we show that altered connectivity after TMS over left DLPFC is preceded by acute co-activation in a specific network (RSN#17). This leads lasting connectivity increase of sgACC to RSN#17 in subsequent RS on a group- as well as on a single-subject level. Notably, a very small number of TMS-pulses (60) led to changes detectable on a single subject level. This setup has the potential to be used to predict treatment success before rTMS therapy plans and adjust important TMS parameters to improve response-rates.