OHBM 2020 – Multi-band accelerated TMS/fMRI for continuous EPI during stimulation shows 10Hz acute effects
Registered OHBM participant? Please rate this abstract here
Martin Tik1, Michael Woletz1, Anna-Lisa Schuler1, David Linhardt1, Allan Hummer1, Christian Windischberger1
1 Medical University of Vienna, Vienna, Austria
Introduction:
Concurrent TMS/fMRI is a challenging field in TMS research and has the potential for revealing brain activity caused by TMS. Among those challenges is the optimal timing between EPI and TMS pulse, since TMS may interfere with the magnetic field inside the MR scanner resulting in severe image artefacts. Different strategies have been introduced for tackling this issue: (1) TMS pulses between the acquisition volumes limiting the repetition rate of pulses to around 1 Hz, (2) sacrificing some slices within the EPI slab and (3) stimulation between EPI slice excitations. Only the latter method allows for high-frequency TMS protocols (10Hz), but requires exact timings of slice acquisitions and TMS pulses to avoid k-space artefacts and ensure good imaging quality. In this study, we optimized imaging parameters for 10 Hz concurrent TMS/fMRI using two 7-channel dedicated RF-coils during a typical protocol used for treatment of treatment resistant depression. By incorporating multi-band acceleration, this is the first study to combine 10 Hz TMS with concurrent full brain coverage EPI acquisition.
Methods:
We used a concurrent TMS/fMRI setup consisting of two 7-channel RF surface coils and an MR-compatible TMS-coil mounted on top of one of the surface coils (MagVenture, Denmark).
Functional images were acquired using the CMRR EPI sequence with TR/TE=1000/38ms, 40 slices, 3 x 3 x 3mm3, 20% slice gap, MB-factor=4. Settings were chosen to force exact slice timing and send slice triggers to the TMS stimulator according to the paradigm. 10Hz TMS trains of 1s with an inter train interval of 9 s were applied at 100% individual resting motor-threshold. Total paradigm length was 800s.
Before in-vivo investigations, we optimized the delay time in a phantom (Figure 1) by increasing the trigger delay on the stimulator in 1 ms steps from 30 to 70 ms in order to avoid artifacts in EPI as well as in k-space data. Optimal delay was determined as 60 ms and was further validated in vivo.
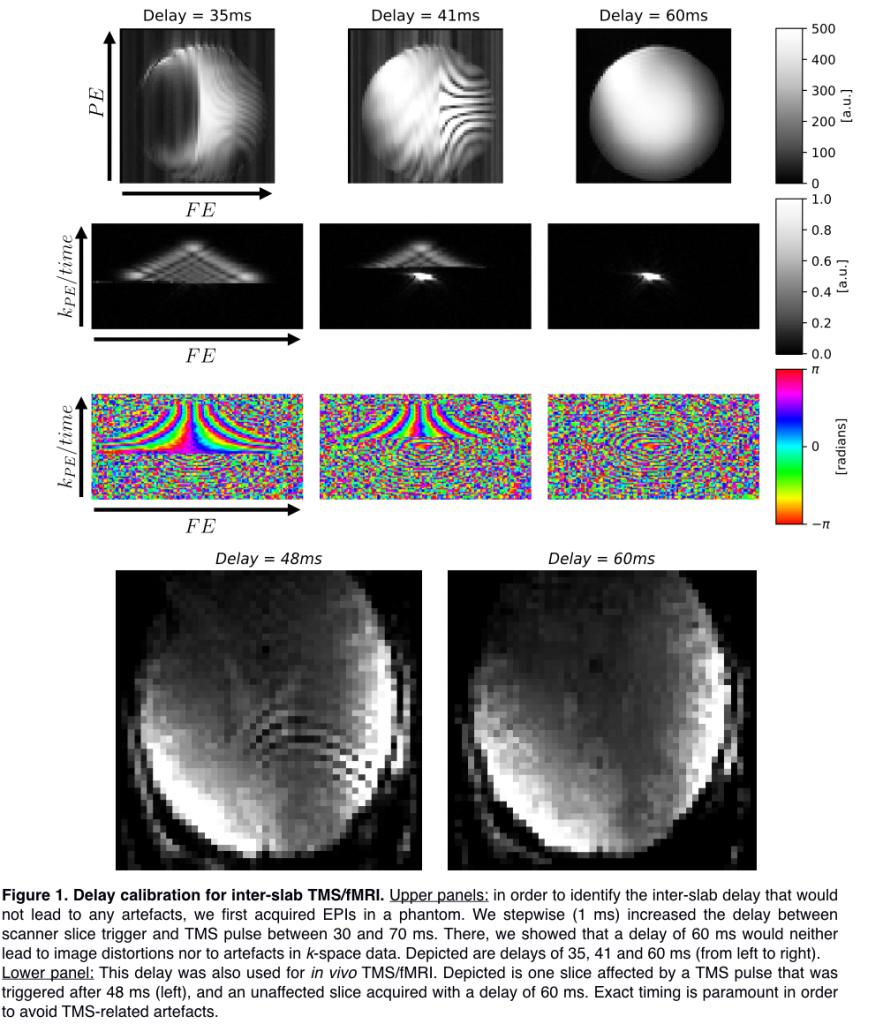
Lower panel: This delay was also used for in vivo TMS/fMRI. Depicted is one slice affected by a TMS pulse that was triggered after 48 ms (left), and an unaffected slice acquired with a delay of 60 ms. Exact timing is paramount in order to avoid TMS-related artefacts.
Results:
Stimulation at 100% of the resting motor threshold led to a significant increase in vmPFC activity (Figure 2 left). This effect is matching the effect of a large resting-state offline TMS study (Figure 2 right), where we demonstrate increased vmPFC connectivity to a specific RSN network after a 10 Hz rTMS protocol.
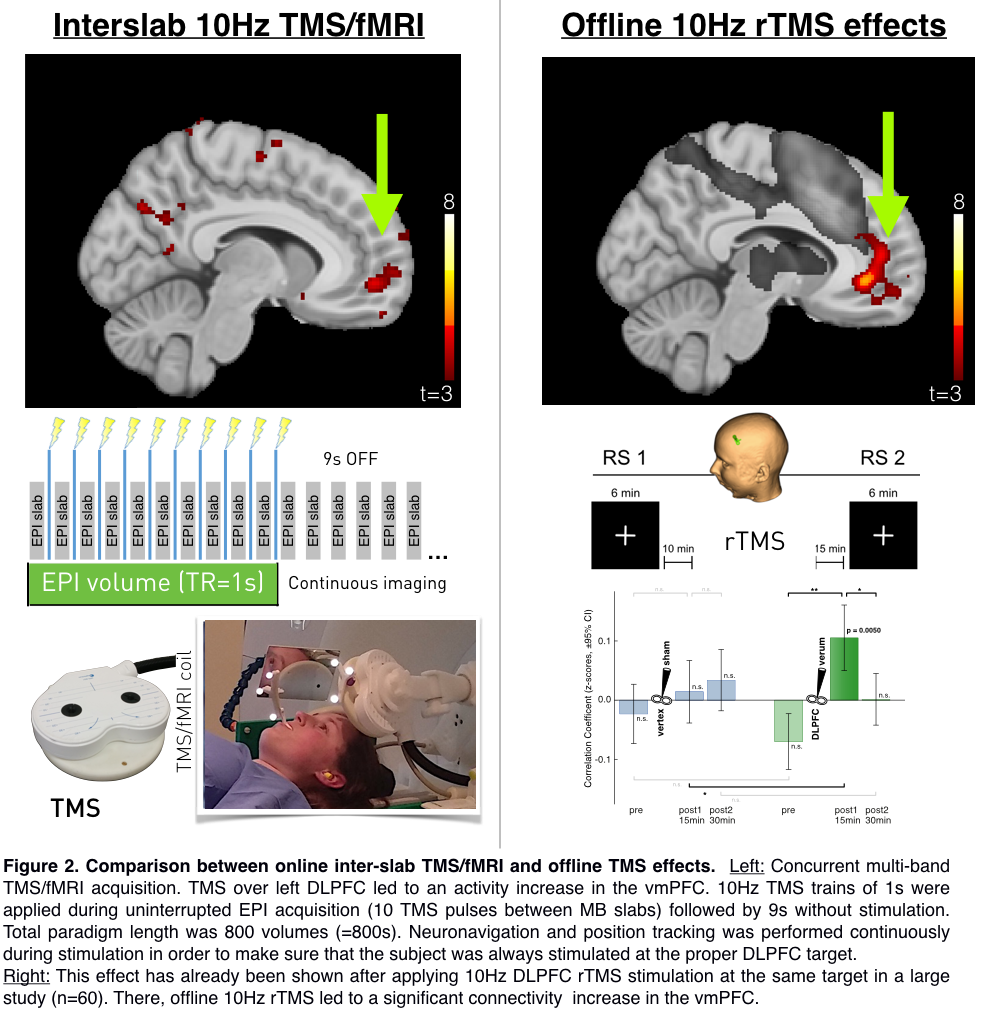
Conclusions:
With this study, we were able to identify MB EPI settings optimal for fast whole-brain image acquisition. Moreover, we validate a concurrent TMS/fMRI protocol that allows for BOLD signal acquisition during a high-frequency 10 Hz protocol without introducing any acquisition gap or artifacts. In this approach we completed a TMS/fMRI procedure that allows for assessing acute 10 Hz TMS protocols, similar to those used in clinical settings. Stimulation of the left DLPFC in this setting resulted in a significant activity increase in the vmPFC, an area that we identified to increase its connectivity after a 10Hz TMS/fMRI protocol (Tik et al., 2017). This new setup for the first time depicts TMS effects of 10Hz stimulation during MB-accelerated EPI without producing image artifacts to guarantee high quality mapping of acute effects.

